[Uni Tübingen] - [Mat.-Nat. Fakultät] - [Fachbereich Chemie] - [Anorg. Chemie] - [Klaus Eichele] - [Software] - [WSolids1] - Dipolar-Chemical Shift

|
WSOLIDS1:
|
Description
This squeezed picture shows an example for the succesful simulation of a spectrum arising from the combined effect of chemical shift anisotropy and homonuclear dipolar coupling in a powder sample. It is the 31P MAS NMR spectrum of tetraethyl diphosphine disulfide. The spectra of static powder samples and an NMR single crystal experiment have been published in:
K. Eichele, G. Wu, R. E. Wasylishen, J. F. Britten:
Phosphorus-31 NMR Studies of Solid Tetraethyldiphosphine Disulfide.
A Reinvestigation of the 31P,31P Spin-Spin Coupling Tensor.
J. Phys. Chem. 1995, 99, 1030-1037.
Click on the picture to have a better look.
Background
In addition to the chemical shift anisotropy (CSA), the spectrum of a spin pair will also depend on the direct dipolar coupling and potentially the indirect spin-spin coupling between both nuclei. Because both, the CSA and dipolar interaction, are tensorial interactions, the actual line shape also depends on their relative orientation.
Examples
The SVG images shown below were produced using the following tools: my own SpecPlot to plot the spectra, Platon to plot the molecular structures from X-ray data, and Inkscape to compose the picture.
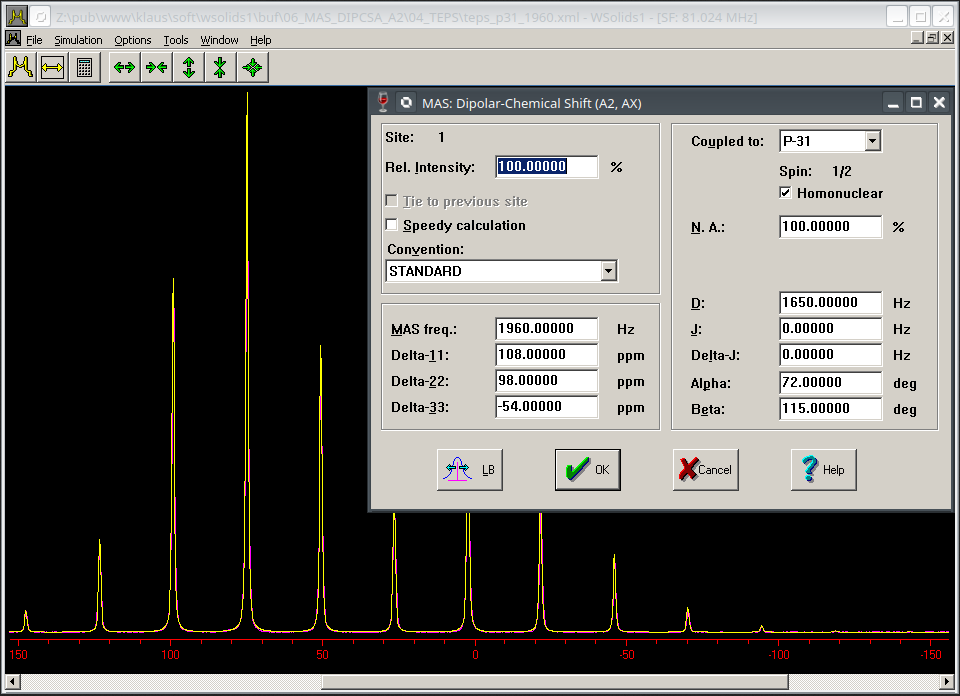
| P-31 CP/MAS NMR spectrum of a powder sample of tetraethyldiphosphine disulfide: the analysis of spectra of static powder samples are shown as examples for Static: Dipolar-chemical shift (A2, AX). The MAS spectrum shown here appears to display chemical shift anisotropy only. However, because we are dealing with an A2 spin system, there is a not so minor effect due to homonuclear dipolar coupling between the two directly bonded phosphorus atoms. This interaction will affect the intensities of the spinning sidebands and falsify a Herzfeld-Berger analysis (for some hints on how to extract the data, see MAS: Chemical shift anisotropy (HB)). The HB analysis gives δ11 = 121.4(0.3), δ22 = 86.5(0.3), δ33 = -56.2(0.2), while the true chemical shift anisotropy is 108, 98, -54 ppm. |
| P-31 CP/MAS NMR spectrum of a powder sample of trimethylphosphine selenide: this example is actually from the literature and illustrates the combination of chemical shift anisotropy, direct dipolar and indirect spin spin coupling to Se-77, a nucleus with lower natural abundance, i.e., there are two isotopologues present. The spin system handles the uncoupled case automatically. The actual example results from the conversion of Fig. 2a in the paper and represents a sample enriched in Se-77 to a level of 70%. The spinning rate given in the figure, 1500, however, is in error, my simulation uses 1300 Hz. This example has been published in G. Grossmann, M. J. Potrzebowski, U. Fleischer, K. Krüger, O. L. Malkina, W. Ciesielski, Solid State Nucl. Magn. Reson. 1998, 13, 71. DOI: 10.1016/S0926-2040(98)00077-0. |
[ Anorg. Chemie ] | [ Go Home ] | webm@ster | last modified: 13.02.2024