[Uni Tübingen] - [Mat.-Nat. Fakultät] - [Fachbereich Chemie] - [Anorg. Chemie] - [Klaus Eichele] - [NMR Ramblings] - [Spin Systems] - AB Spin System
 |
Spin System: AB, General Information |
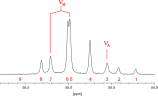
Appearance of an AB NMR Spectrum
|
|
|
|
- The spectrum of an AB spin system consists of 4 peaks.
- The analysis gives |JAB|, and D (difference of chemical shifts of A and B, in Hz).
- JAB appears two times in the spectrum (property of repeated spacings), as outer separation of peaks.
- The intensities should correspond to the intensity sum rule:
I1 = I4, I2 = I3; I1 + I2 = I3 + I4 - Consequences of the AB character are:
- the intensity ratio of stronger (inner) peaks to weaker (outer) peaks depends on the
ratio JAB to D ("roof effect"), i.e., the multiplets "lean" towards each other:
I2 / I1 = I3 / I4 = (ν1 - ν4)/(ν2 - ν3) - the true chemical shifts of A and B are no longer at the center of the A- or B-doublet, but shifted towards the stronger inner peak. (Actually, the doublets move away from the center of gravity f0) The error in chemical shifts, if picked from the centers of the doublets, corresponds to (2C-D)/2, see example below.
- The ratio D/J is used to draw the line between AB and AX spin systems: if the ratio is less than 10, the spin system is called AB, if the ratio is greater than 10, the spin system is called AX, although intensity deviations may still be visible (for D/J = 10, I2/I1 = 1.16).
- The above expressions for the transition energies and intensities are used on the page
on
AB spectrum simulation. - The analysis of an AB NMR spectrum can be performed interactively on the page
on
AB spectrum analysis.
Additional Information on the AB NMR Spectrum
-
A series of nice examples is featured on Hans Reich's website,
while a useful method to find the "leaning partner" B, if A is given, is illustrated on
Stans Blog.
However, I disagree in one point: I would call the AB pattern neither a quartet nor a quadruplet, but two doublets, because those are the multiplets they will form if the external magnetic field is sufficiently strong. For the same reason, no one would call the H-1 NMR spectrum of ethanol an octet or octuplet, but a singlet and quartet and triplet. (N.B. I was not the cause of the revision mentioned on Stans Blog!) -
Another nice example of how the AB doublets are shifted away from their common center of gravity and hence from
the "true" chemical shift position is provided by the Sn-119{H-1} NMR spectrum of Jens Henning's unysmmetrical
1,2-distanna-cyclobet-3-en (compound 8 in ref. [4]). The 186.50 MHz NMR spectrum consists of several
isotopologues:
- there are two strong peaks corresponding to the situations that there is one Sn-119 nucleus at the A (-9.8 ppm) or B (-92.0 ppm) positions
- if, in addition to Sn-119 at A, there is a Sn-117 nucleus present at B, a doublet of satellites appears
symmetrically about A, and vice versa for Sn-119(B) with a symmetrical doublet of satellites caused by Sn-117 at A.
(In principle, there could be a small isotope shift that would displace the center of the doublet from the position of the uncoupled Sn-119, but this effect is too small in the present case).
Because Sn-119 and Sn-117 are different nuclei, the Sn-119,Sn-117 isotopologue constitutes a heteronuclear AX spin system.
(If the J(Sn-119,Sn-117) is sufficiently great, even this heteronuclear system could form an AB spin system at low magnetic fields, see ref. [5] for an example.) - in contrast, if the second nucleus is also Sn-119, the spin system is homonuclear and forms an AB spin system, because J(Sn-119,Sn-119) = 7037.4 Hz and D = 15338.0, hence D/J = 2.18, smaller than 10. The difference between the center of the doublet and the true chemical shift is (2C-D)/2 = 768.7 Hz or 4.1 ppm. This AB spectrum provides the default values on the page on AB spectrum analysis.
References
- Günther, H. NMR-Spektroskopie, Eine Einführung. Georg Thieme Verlag, Stuttgart, 1973.
- Garbisch, E. W., Jr., J. Chem. Educ. 1968, 45, 402–416, DOI: 10.1021/ed045p402.
- Dischler, B. Angew. Chem. 1966, 78, 653–663, DOI: 10.1002/ange.19660781302;
Angew. Chem. Int. Ed. Engl. 1966, 5, 623–633, DOI: 10.1002/anie.196606231. - Schneider, J.; Henning, J.; Edrich, J.; Schubert, H.; Wesemann, L. Inorg. Chem. 2015, 54, 6020-6027, DOI: 10.1021/acs.inorgchem.5b00896.
- Lassigne, C. R.; Wells, F. J.; Farrugia, L. J.; James, B. R. J. Magn. Reson. 1981, 43, 488-490, DOI: 10.1016/0022-2364(81)90062-7
[ Anorg. Chemie ] | [ Go Home ] | webm@ster | created: 02.01.2013 | last modified: 06.07.2017

