[Uni Tübingen] - [Mat.-Nat. Fakultät] - [Fachbereich Chemie] - [Anorg. Chemie] - [Klaus Eichele] - [NMR Ramblings] - NMR of Acetanilide
|
NMR of AcetanilideContents: [ Introduction ] [ Structure ] [ H-1 NMR ] [ C-13 NMR ] [ Links ] |
Introduction
The log files of this web server indicate that a lot of people come to my pages because they were searching for "NMR of acetanilide" or similar. They got directed to my list of publications because two of my papers dealt with solid-state NMR of acetanilide or N-methylacetanilide. I don't suppose that they found what they were looking for! Although I don't know what those people expected to find I have created this special "NMR of acetanilide" page as a service to the Web community. If this page shows what you wanted, or you were looking for something else, why don't you let me know?
Structure of Acetanilide
If your browser supports or allows JavaScript, you can view and manipulate a molecular view
of acetanilide on my
Structure of Acetanilide page
The crystal structures of acetanilide have been published in:
S. Johnson, J. Eckert, M. Barthes, R. Mc Mullan,
"Crystal Structure of Acetanilide at 15K and 295K by neutron diffraction",
J. Phys. Chem. 1995, 99, 16253
https://doi.org/10.1021/j100044a009
H. J. Wasserman, R. R. Ryan, S. P. Layne,
"Structure of acetanilide (C8H9NO) at 113 K",
Acta Cryst. 1985, C41, 783.
http://dx.doi.org/10.1107/S0108270185005492
You can download a file containing the coordinates from the Wasserman paper in either PDB or CIF format. If you don't have software to view structures, you may want to try some of the programs listed on my X-ray links page. Or visit my Structure of Acetanilide page mentioned above.
H-1 NMR Spectrum of Acetanilide
The following chemical shifts were reported [1,2] for the protons of acetanilide:
| CH3 [1] | 2.1 | 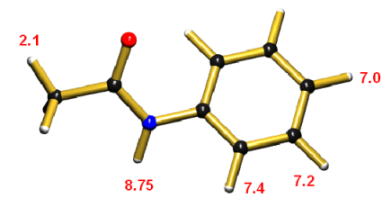 |
| para-H [1] | 7.0 | |
| meta-H [1] | 7.2 | |
| ortho-H [1] | 7.4 | |
| NH [2] | ca. 8.75 |
| [1] | M. Hesse, H. Meier, B. Zeeh: Spektroskopische Methoden in der organischen Chemie, Georg Thieme Verlag, Stuttgart, 2nd ed., 1984, p. 263. |
| [2] | from a H-1 NMR spectrum shown by
Stephen Jones |
C-13 NMR Spectrum of Acetanilide
The following chemical shifts were reported [1] for the carbons of acetanilide:
| CH3 [1] | 24.1 | 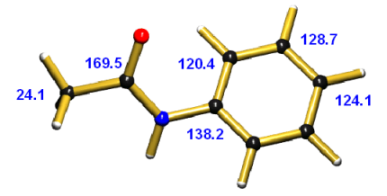 |
| para-C [1] | 124.1 | |
| meta-C [1] | 128.7 | |
| ortho-C [1] | 120.4 | |
| ipso-C [1] | 138.2 | |
| CO [1] | 169.5 |
| [1] | M. Hesse, H. Meier, B. Zeeh: Spektroskopische Methoden in der organischen Chemie, Georg Thieme Verlag, Stuttgart, 2nd ed., 1984, p. 263. |
| [2] | there is a C-13 NMR spectrum shown by
Stephen Jones |
Links and References in Crude Form
NIST entry of physical propertiesAga, D.S., Rentsch, D., Hany, R. and Muller, S.R.: Sulfonic and Oxanilic Acid Metabolites of Acetanilide Herbicides: Separation of Diastereomers and Enantiomers by Capillary Zone Electrophoresis and Identification by 1H NMR Spectroscopy Environ. Sci. Technol. 1999, 33, 3462-3468: https://doi.org/10.1021/es990288w
M. D. Morton, F. H. Walters, D. S. Aga, E. M. Thurman, and C. K. Larive: Nuclear Magnetic Resonance Identification of New Sulfonic Acid Metabolites of Chloroacetanilide Herbicides Journal of Agricultural and Food Chemistry, 1997, 45, 1240-1243: https://doi.org/10.1021/jf960453g M.Barthes, M. Ribet "Compared 13C NMR spectra of Acetanilide and N-methylacetamide", Berichte der Bunsengesellschaft, 1997, 102(3), 419-421: https://doi.org/10.1002/bbpc.19981020321 M.Barthes, G.De Nunzio, M. Ribet, "Polarons or proton transfer in chains of peptide groups", Synthetic Metals v.76,337, (1996): https://doi.org/10.1016/0379-6779(95)03484-2 Johnson, M. R., Prager, M., Grimm, H., Neumann, M. A., Kearley, G. J. and Wilson, C. C.: Methyl group dynamics in paracetamol and acetanilide: probing the static properties of intermolecular hydrogen bonds formed by peptide groups. Chemical Physics, 1999, vol. 244, 49-66: https://doi.org/10.1016/S0301-0104(99)00138-X A. Bulai, A.-A. Alencar de Queiroz, A. Gallardo and J. San Roman: Microstructural analysis of p-acryloyloxy-acetanilide and N,N-dimethylacrylamide copolymers of biomedical interest by NMR POLYMER, VOL. 40 , NO. 17 , 1999, 4953 https://doi.org/10.1016/S0032-3861(98)00710-1
[ Anorg. Chemie ] | [ Go Home ] | webm@ster | last modified: 19.03.2020

