[Uni Tübingen] - [Mat.-Nat. Fakultät] - [Fachbereich Chemie] - [Anorg. Chemie] - [Klaus Eichele] - [NMR Ramblings] - [Spin Systems] - ABX Spin System
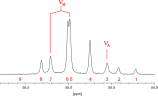 |
Spin System: ABX Simulation | AB Part |
Input Section
Please enter the three coupling constants (in Hz), the chemical shifts of A and B (in ppm), the corresponding frequency SF of the reference compound (in MHz) as well as the desired total width of the simulated spectrum in pixels (be reasonable ;-). The simulation is based on the exact transition energies and intensities reported by Garbisch [2]. To prevent a division by zero problem if J(AB) = 0, its value will be set to 0.00001 Hz internally.
Simulation of ABX
| vA: -509.65 Hz | δA: -5.0333 ppm vB: -860.65 Hz | δB: -8.4997 ppm first AB spectrum (red): v1' = 44.67 | v2' = -105.60 | v3' = -603.37 | v4' = -753.64 I1' = 0.7681 | I2' = 1.2319 | I3' = 1.2319 | I4' = 0.7681 second AB spectrum (blue): v1'' = -857.45 | v2'' = -1007.72 | v3'' = -1023.91 | v4'' = -1174.18 I1'' = 0.0973 | I2'' = 1.9027 | I3'' = 1.9027 | I4'' = 0.0973 | X part (magenta):
| ||||||||||||
References
- Günther, H. NMR-Spektroskopie, Eine Einführung. Georg Thieme Verlag, Stuttgart, 1973.
- Garbisch, E. W., Jr., J. Chem. Educ. 1968, 45, 402–416, DOI: 10.1021/ed045p402.
- Dischler, B. Angew. Chem. 1966, 78, 653–663, DOI: 10.1002/ange.19660781302;
Angew. Chem. Int. Ed. Engl. 1966, 5, 623–633, DOI: 10.1002/anie.196606231.
[ Anorg. Chemie ] | [ Go Home ] | webm@ster | created: 02.01.2013 | last modified: 05.04.2024

